Abstract
Ruxolitinib (rux) was originally approved for treatment of symptomatic patients with advanced myelofibrosis due to its significant activity shrinking spleen size and reducing cytokine-driven symptom burden (1). Rux has since received approval as second line therapy for polycythemia vera (2, 3) and is in clinical trials for essential thrombocythemia (4). Accordingly, rux use among patients with Philadelphia-chromosome negative (Ph-) myeloproliferative neoplasms (MPNs) is increasingly common. Weight gain among cachectic patients is thought to be a beneficial effect of rux therapy in this patient population (1, 5), but its underlying mechanism on body weight has not been studied.
Rux is a JAK1/2 tyrosine kinase inhibitor that blocks both normal and pathogenic JAK/STAT signaling via receptors that utilize these adapter proteins. Leptin (LEP) signaling is part of a complex homeostatic mechanism regulating appetite, metabolism and body weight. In animal models, disruption of LEPR-mediated JAK2 activation in the ventral-medial hypothalamus (VMH) phenocopies LEPR disruption (6) thereby implicating JAK2 inhibition in body weight homeostasis. Our study aimed to investigate the role of rux on JAK/Stat signaling in mouse brain.
We identified 79 patients with Ph-MPNs treated with rux at Weill Cornell Medicine by Silver MPN Center physicians. We identified baseline demographics including age, gender, diagnosis, date of diagnosis, transformation, height, weight, body mass index (BMI), systolic and diastolic blood pressure, Lipid profile, HGBA1C, glucose, and diabetic and hypertensive medications at the start and during ruxolitinib therapy. Body weight, BMI and effects on glucose, lipids and blood pressure were assessed during rux therapy.
To assess the effect of rux on VMH LEPR signaling, 8-week old male C57BL6/J mice were divided into 3 groups: Fasted (overnight), Fed, and Fed treated with rux. Rux (60mg/kg) or vehicle control was administered by gavage the day prior to perfusion. Mice were perfused with PFA 4% and brains were cryopreserved. Stat3 phosphorylation was used to report VMH Lepr activation.
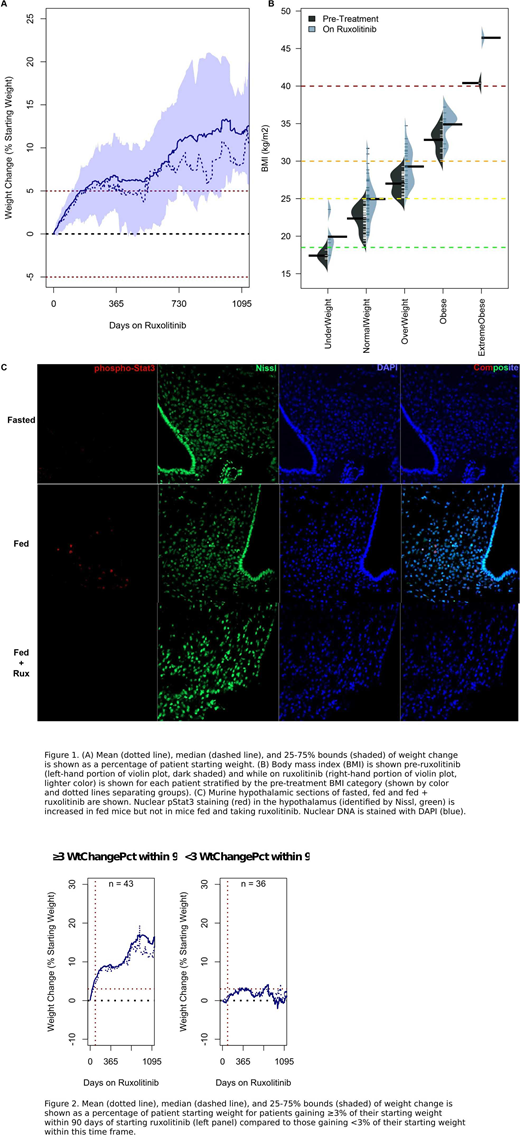
MPN patients received rux for a median of 80 weeks (range: 3.2-243) during which 64 (81.0%) patients gained weight and 29 (36.7%) gained more than 10% of their pre-treatment weight (Figure 1A). On average, patients gained 8% of their starting weight while on rux. Weight gain among those gaining weight ranged between 3% and 38% of their starting weight and the median weight gain was 9.5%. MPN diagnosis was not a good predictor of weight gain (Figure 1B) nor was pretreatment BMI. Indeed, only a small portion of the treated patients were underweight at the start of therapy and, within each BMI category, the majority of patients gained significant weight. We found that total cholesterol and LDL cholesterol were higher among patients whose BMI increased by more than 5% while on ruxolitinib therapy. Contrary to expectations, blood glucose, HgbA1c and blood pressure were not increased in patients with more than a 5% increase of their starting BMI. Because significant weight gain could contribute to increased risk of cardiovascular disease and other co-morbidities, we searched for a simple, clinically useful predictor to identify patients most likely to gain significant weight (> 10% starting weight), become obese or move to a higher BMI category while on rux therapy. We found that patients gaining more than 3% of their pre-treatment weight within 90 days of starting rux were destined to continue gaining weight while on therapy (p<0.005, Fig2).
Mechanistically, single dose of rux decreased LEPR signaling in the VMH region in fed animals reducing signaling to levels comparable to that seen in fasted mice. In contrast, fed control mice showed robust VMH phosphor-Stat3 (Fig1).
A large proportion of patients with Ph-MPNs gain considerable weight while receiving treatment with rux. Weight gain is a general phenomenon and not restricted to cachectic patients. Patients who gain ≥3% of their pre-treatment weight during the 3 months of rux therapy are destined to gain significant weight while on therapy. These patients should receive appropriate dietary counseling and lifestyle management recommendations to help mitigate this outcome. In addition, our animal studies support the hypothesis that rux blocks normal homeostatic LEPR signaling and could reduce post-prandial satiety thereby leading to hyperphagia and weight gain.
Ritchie:Bristol-Myers Squibb: Research Funding; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Pfizer: Consultancy, Research Funding; Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau; ARIAD Pharmaceuticals: Speakers Bureau; Astellas Pharma: Research Funding; NS Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal